Latest Event Updates
Building The Rangoonwala Terrace
We will be moving our blog soon to a combined natural history themed site. For more future content, please follow https://naturemanchester.wordpress.com/ ……….
The plantings on the terrace are the result of research carried out during the Museum’s hello futuredevelopment project. Gardening on roof spaces creates very particular challenges, and we are grateful to our friends at the National Trust Castlefield Viaduct for their help and advice on watering and planting schemes, but most especially about growing mediums. The plants here are planted in a lightweight mix including peat-free compost, fine-milled composted bark, perlite and expanded clay granules (hydroleca).




The Rangoonwala Terrace will continue to develop over time, and by using wooden troughs and RootPouch plant containers we can rearrange the plant groupings as plants grow and change. Over the seasons, plants will be moved to bring in different seasonal interest and to rest some when necessary. Having access to the University’s…
View original post 206 more words
The Rangoonwala Terrace
We will be moving our blog soon. For more content in future, please follow https://naturemanchester.wordpress.com/ ……….
For those who have visited Manchester Museum since we reopened in February this year, you will have spotted The Rangoonwala Terrace, the lightwell garden space sitting between the South Asia Gallery and the Lee Kai Hung Chinese Culture Gallery. Over the changing seasons, we will be posting a series of plant profiles highlighting stories from the plants in this space.
This shaded terrace hosts plants such as primroses, hardy geraniums, and pheasant berry that come from the Himalaya mountains. While the damp and changeable British weather is not ideal for plants from South Asia, species like rhododendrons are familiar additions to our parks and gardens. The plants that thrive here are often from similar climates in mountainous regions.
The range of climates and landscapes across South Asia encourages very diverse plant life. Three biodiversity hotspots – the Western Ghats, Sri Lanka and the Himalayas – have unique plant species found…
View original post 56 more words
City Nature Challenge 2023: Greater Manchester

Friday 28th April – Monday 1st May 2023
The City Nature Challenge is back for 2023. Spot the wildlife on your doorstep this spring and help put nature on the map in Greater Manchester. We need you to get out and about in your local parks and greenspaces looking for the wildlife which we share our city with.
Join residents from 400+ cities across the world for the 6th international City Nature Challenge, an epic global mission to record as much wildlife as possible over four days in spring. Find out what exciting plants, animals and fungi live in your local area. The first step towards protecting nature in our local environments is finding our what already lives there.

Over the four-day challenge, people across the UK – and the world – will be coming together to share observations of nature using the free iNaturalist app. You…
View original post 149 more words
Manchester Festival of Nature 2021: Pollinator Portraits competition
Manchester Festival of Nature (MFoN), Sunday 27th June 2021
It’s been a strange time for festivals and events. Following 2019’s scorchingly hot event at Heaton Park, 2020’s Festival of Nature went entirely online with some great digital webinars and workshops, oh, and me with my first attempt at live-streaming some plant pressing. Best leave it to the professionals!
This year we again have some wonderful online content for everyone to enjoy, and we started early this year with twitter takeovers throughout the month of June.
https://twitter.com/i/events/1391444989322874883
On the day of the Festival, you can get to all the new content on our ‘main stage’ twitter account @MancNature. Also, if you are lucky enough to be taking a Sunday stroll through Heaton Park today, you might spot the Lancashire Wildlife Trust’s gazebo and chat to them about wildlife in the park.
This year, the Museum has supported the Manchester Nature Consortium Youth Panel to deliver their art competition for the Festival. Taking inspiration from the bee and insect themes of the Festival, the young people launched a Pollinator Portrait competition earlier this year. They must have faced a tough choice voting for the winners as the quality of entries was really good. The Youth Panel loved the Museum’s Beauty and the Beasts digital exhibition, so we decided to create an online exhibition to display all the works.
Head on over to our online gallery to see for yourself……..
Good wildlife spotting everyone!
With everyone staying close to home, this year the wildlife spotting for the City Nature Challenge has been really urban. If you have more images taken over the weekend, you can still upload them now into iNaturalist and your sighting will be added into the count. Otherwise, it’s time to try and identify all those finds! Let’s see how many we can push to be research grade records.
I suspect we’ve had far more pavement weeds this year than we did last year. Certainly, last year the top three organisms recorded where blackbirds, harlequin ladybirds and wood pigeons. So far this year, our top three are cuckooflowers, Herb Robert and dandelions. Of course, although the weekend of wildlife spotting is over, we’ve now got time to make sure as many records as possible are properly identified, so that list could change.
Happily, although everyone was limited to gardens and short walks, the weather was much kinder than last year allowing us to really enjoy our local wildlife. There have been plenty of bee and butterfly garden visitors and the occasional bird to watch as well as all the plants. If you have enjoyed a weekend of wildlife recording, check out Greater Manchester’s Local Record’s Centre so that you can continue putting nature on the map. There’s also advice from the Wildlife Trust for Lancashire, Manchester and North Merseyside on how to improve your garden for wildlife. Click here to apply for a free downloadable booklet from the My Wild City Manchetser project.
The City Nature Challenge weekend has been popular across the country with over 4,000 people taking part and just under 60,000 observations made. If know of a city or region that would want to take part next year, then get in touch with the organisers. The City Nature Challenge was invented and is managed by the Natural History Museum of Los Angeles County and California Academy of Sciences: https://citynaturechallenge.org/
City Nature Challenge 2020 in lockdown
Last year Greater Manchester competed in the City Nature Challenge for the first time. City Nature Challenge was started by the Natural History Museum of Los Angeles County and California Academy of Sciences in 2016, and it has grown into a world-wide urban nature event. The challenge aims to get people around the world involved in wildlife recording and learning about nature in their local patch. It uses the wildlife app iNaturalist, which is great for people .
This year, however, with the world on lockdown things are a little different. Rather as a challenge with competing cities, this year is a celebration of the nature that we have living all around us. Spending time with nature has been shown to help our mental health, so this weekend, why not join us for the #CityNatureChallenge? Follow social distancing guidelines and try some birdwatching from the windows, spot the spiders in the cupboards, identify the insects visiting the garden or windowboxes, and share the plants you see in your local streets. You can also go online to help identify other people’s finds.
You might just find yourself catching the wildlife recording bug!
‘Beauty and the Beasts’ – celebrating insects
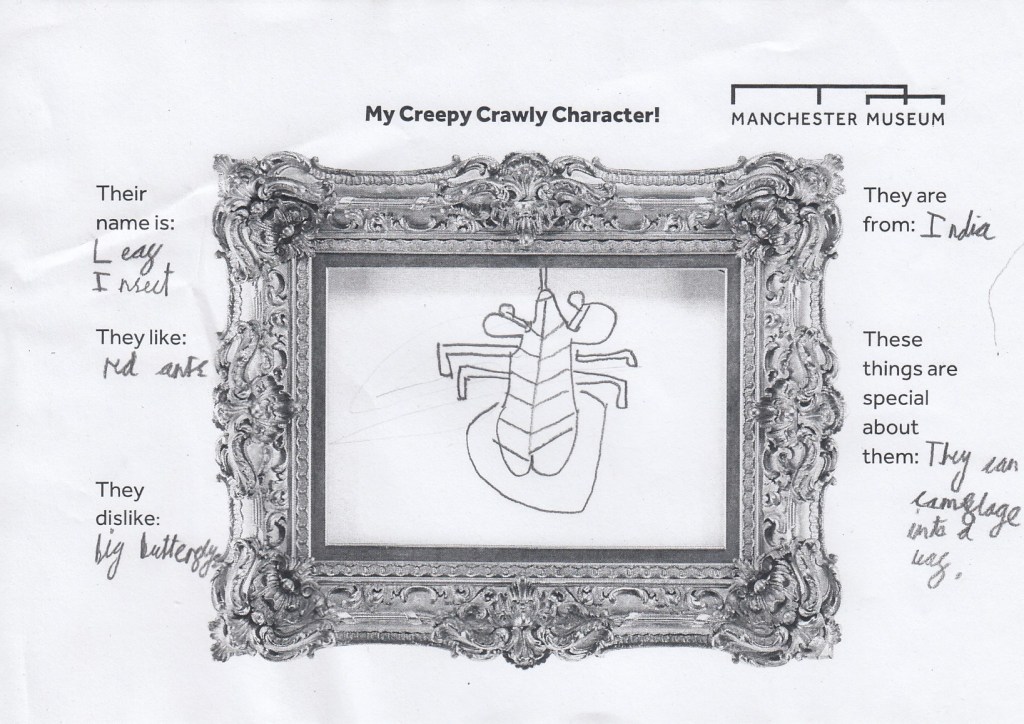
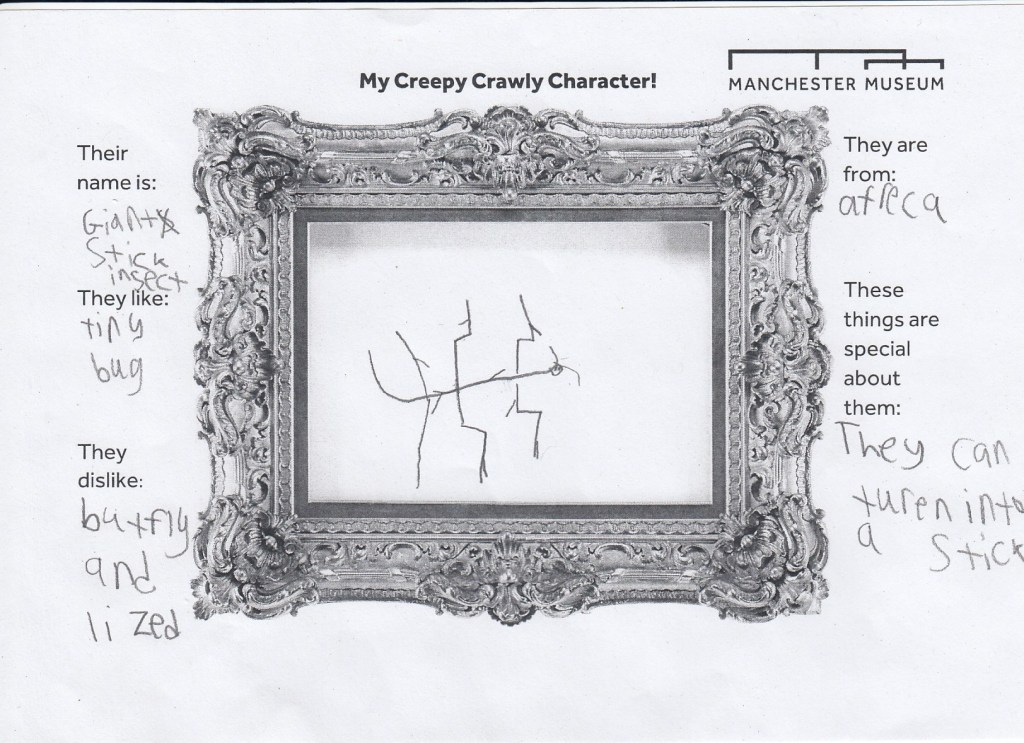
In the last few months many activities have taken place in and around our temporary exhibition on the top floor of Manchester Museum, ‘Beauty and the Beasts; falling in love with insects’. For example, children and young people, from under 5s to teenagers, were invited to send stories involving insects to help us create the next great Creepy-Crawly Chronicle, following in the footsteps of the Hungry Caterpillar. There is still time to enter the Children’s Story Competition, see here for more information.

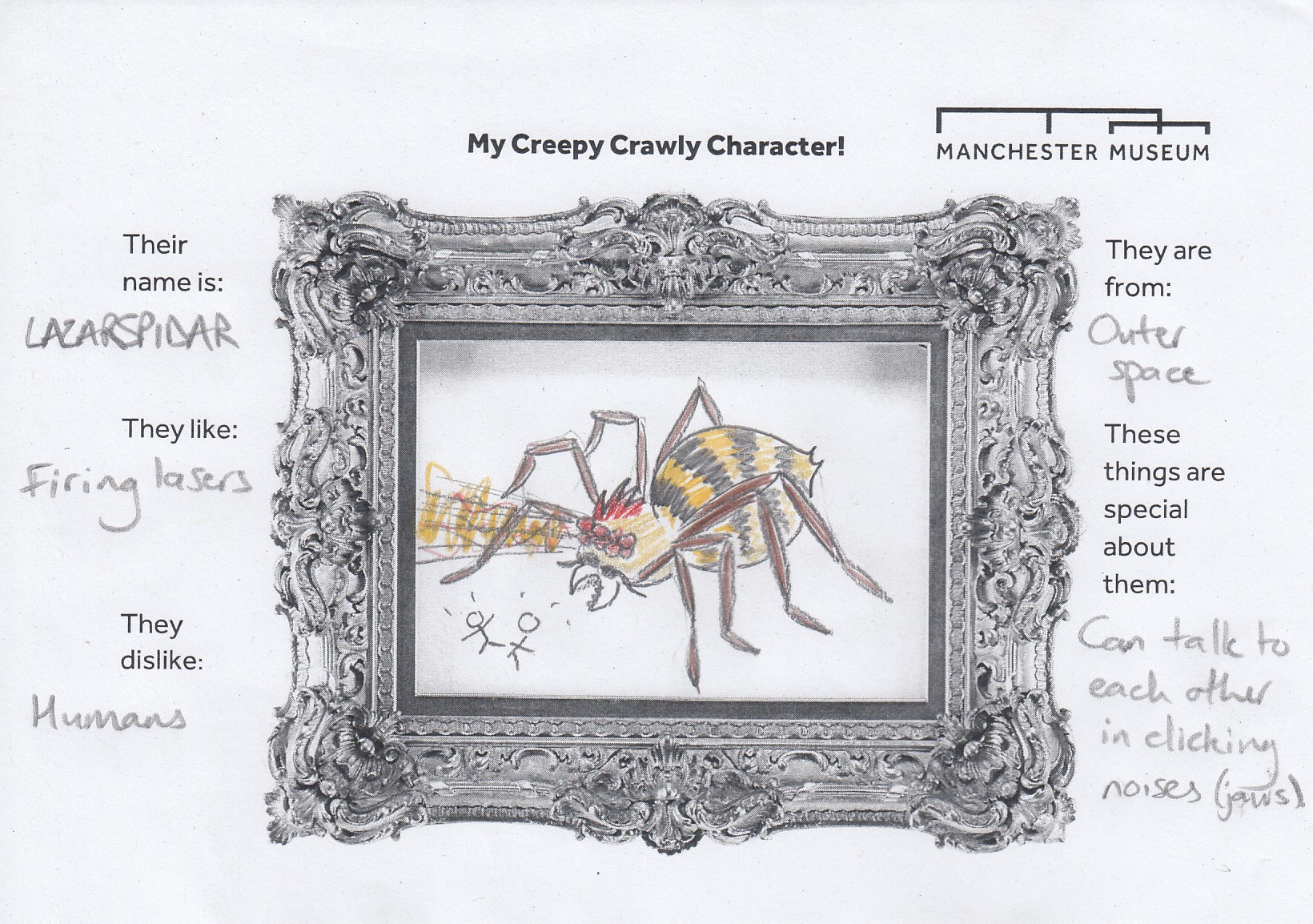
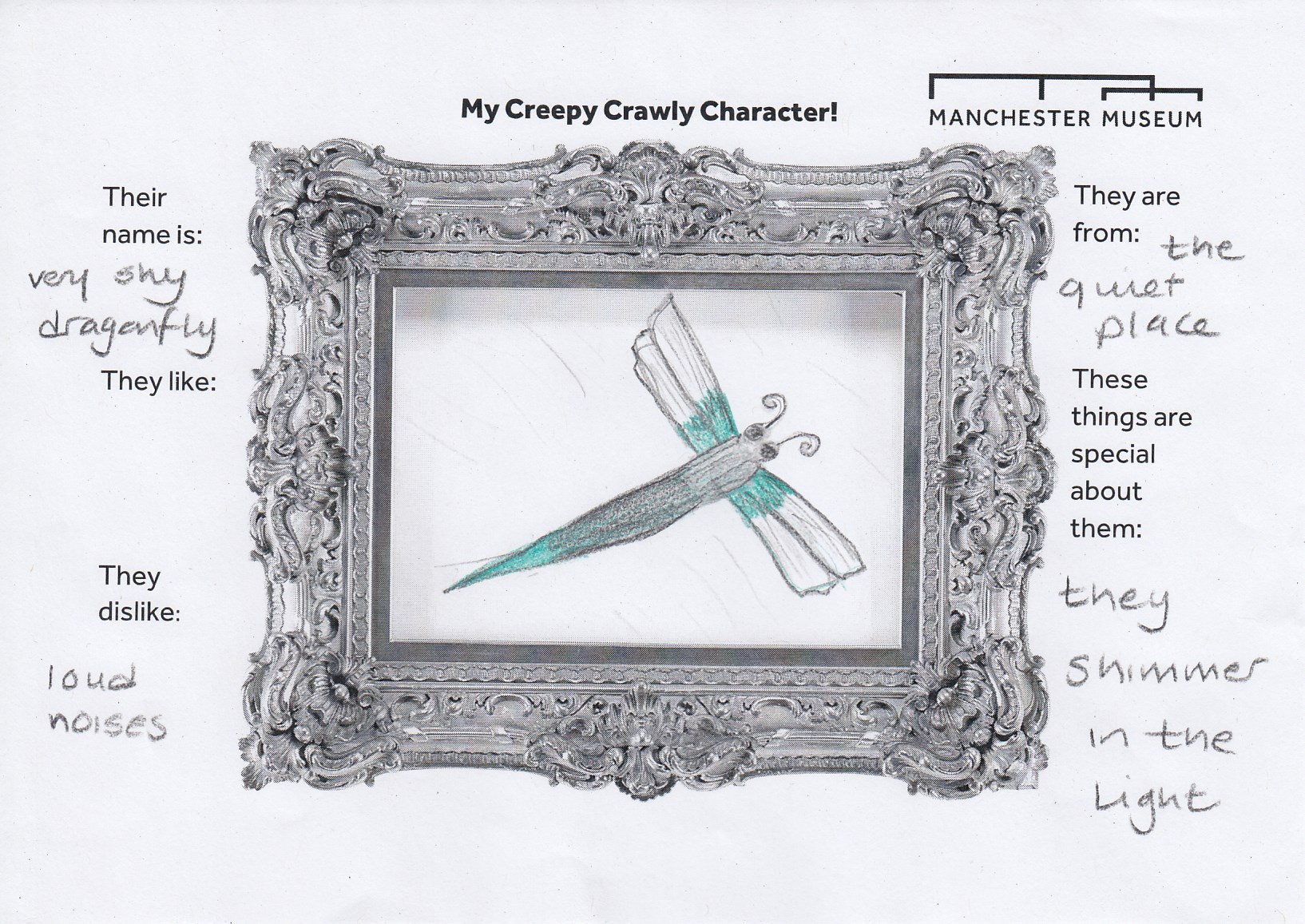
Inspired by the amazing creatures in Beauty and the Beast Exhibition and the insects on the handling table, younger visitors created their very own creepy-crawly characters.
The exhibition has also been used as a space to relax and enjoy, for example, hosting one of the wellbeing sessions for Natural Sciences students at the University of Manchester…
View original post 246 more words
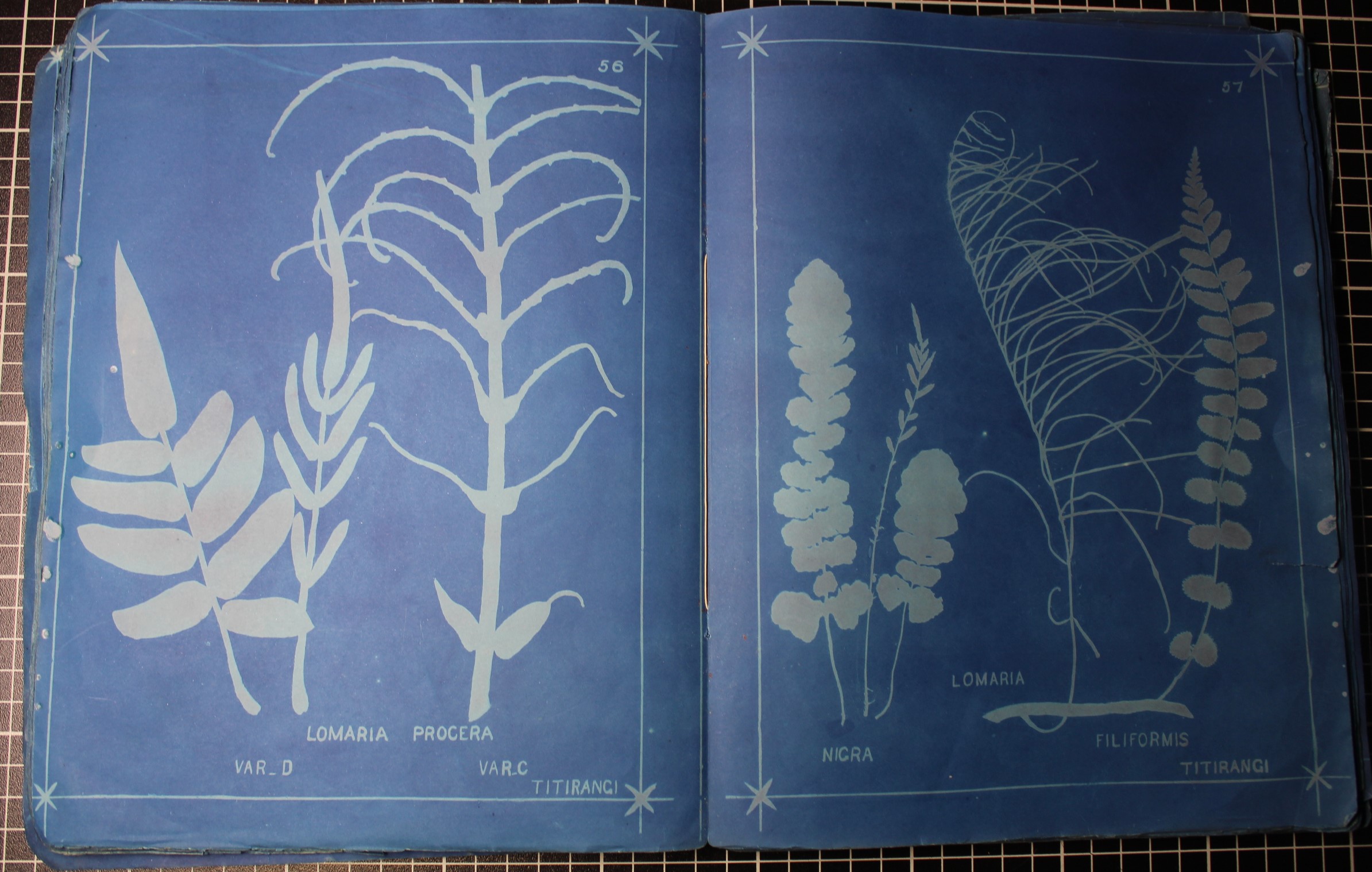
A Christmas Craze – The Fern Madness!
 A Victorian Christmas card in our Nature’s Library Gallery, Manchester Museum
A Victorian Christmas card in our Nature’s Library Gallery, Manchester Museum
The Fern Madness!
“I wish it could be Christmas, every day…” So goes the popular song, but at Manchester Museum we do have a Christmas card on display 365 days a year, in the Nature’s Library gallery.
But what plants are those? Are they herbs for Christmas dinner? Are they plants grown in the house over Christmas? No, not at all – these are ferns, and they are here as a remnant of the Pteridomania or ‘Fern Madness’ that swept the nation in the Victorian era. The term ‘Pteridomania’ was coined by Charles Kingsley in 1855:
“Your daughters, perhaps, have been seized with the prevailing ‘Pteridomania’…and wrangling over unpronounceable names of species (which seem different in each new Fern-book that they buy)…and yet you cannot deny that they find enjoyment in it, and are more active, more cheerful, more…
View original post 365 more words